In the pharmaceutical industry, the BMR is usually a element of fine Production Tactics (GMP) and aids be certain that every batch is created in a very controlled and dependable manner.
In other words, how you make anything helps to outline its degree of high quality. Blocking problems is more practical than finding rejects as it is not possible to detect all rejects.[2] The present requirement for ’documented evidence’ can be driven by this event of Devenport.
Any amendments need to be formally licensed and signed by competent human being(s). The amended document need to be replaced at the earliest opportunity by a newly geared up grasp formula.
ICH E6 (R2) is a world guideline that specifies excellent clinical follow for scientific trials involving humans.
Stage two documents shouldn't present specific directive Guidelines or sorts for documenting data but somewhat supply the overall intentions and rules governing crucial courses or systems in addition to explanation to the rationale and plan types. These documents will use to all departments within a GMP-compliant organization.
Documentation: A QA human being is to blame for making sure that each one vital documentation is finished accurately, in the well timed method, As well as in compliance with regulatory demands.
Pharmaguideline can be a pharmaceutical blog site where pharmaceutical principles are stated in very simple and easily comprehensible language for gurus and website pupils. All articles or blog posts and SOPs are created by Ankur Choudhary.
Payment plans are available to those who qualify. Alternatively, purchasing person programs one after the other is likewise an option.
Concluded merchandise testing: QC gurus inspect and approve items to be certain they meet the demanded customer and regulatory standards.
The following checkpoints/checklist may perhaps support to assess the compliance of ‘documentation and records’ with GMP demands
With a transparent idea of GDP rules in addition to a dedication to finest practices, companies can reach regulatory compliance though creating a foundation of have confidence in and accountability.
A particular approach can systematically develop an item that satisfies its predetermined specs and top quality attributes. Approach validation can be a part with the validation, which is explained beneath.
Common running strategies (SOPs) are documents that provide Instructions regarding how to accomplish distinct procedures. These SOPs are utilized by pharmaceutical companies to guarantee consistency and compliance inside the execution of jobs.
The chain of functions that compromised the protection on the drug products integrated insufficient maintenance, inadequate idea of autoclave Procedure, and typical deviations from get more info your written production Guidelines (usually as an try and compensate for tools malfunction). With each other, these factors resulted inside a sterilization cycle that did not assure that every one vials while in the autoclave have been sterilized; Therefore, some doses ended up Harmless, while some led to sepsis in individuals who obtained them.
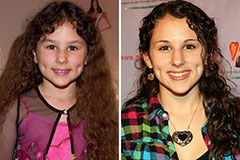 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Mackenzie Rosman Then & Now!
Mackenzie Rosman Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now! Robin McGraw Then & Now!
Robin McGraw Then & Now!